Lobulated, fungating, red-blue lesion on the left posterior maxillary alveolar ridge
Can you make the correct diagnosis?
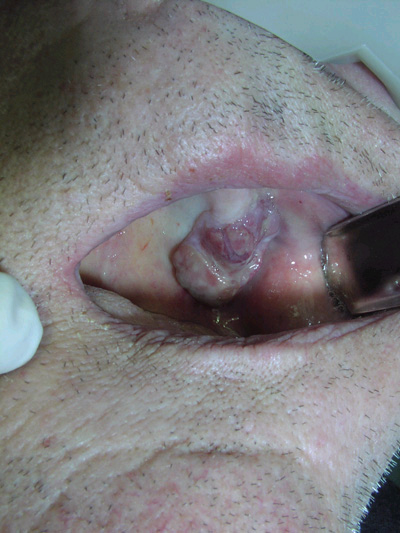
This is a 58-year-old white male presenting with a slow-growing lobular, exophytic, red-blue fungating lesion on the left posterior maxillary alveolar ridge.
Sorry! you are incorrect
Given the patient’s chronic and heavy cigarette use over the past forty years, squamous cell carcinoma should be included in the differential diagnosis. Clinical presentation of SCC emerging from a non-healing ulcer, although not common, is fairly well described in SCC of the gingiva. Oral squamous cell carcinoma is a highly aggressive neoplasm that currently ranks as the fifth most common malignant neoplasm worldwide and accounts for 90% of oral malignancies (1). The incidence of oral SCC is incessantly increasing. For details on SCC, please read the April 2004 Case of the Month. The five-year survival rate ranges between 35% and 50% (2) and has not significantly improved despite increasingly sophisticated medical accomplishments. Oral SCC occurs predominantly in males over the age of 40 years, with an observed male to female ratio of 1.4:1 in the USA (1). Excluding the outer lip, the most common sites (in decreasing order) are ventral and lateral surfaces of tongue (25-50%), floor of mouth (15%), gingiva (12%) and palate (9%). The buccal mucosa and retromolar pad areas (3%) have a relatively low incidence of occurrence (3). Gingival SCC has a tendency to destroy bone in about 50% of cases. Oral SCC varies in presentation from deceptively innocent looking to obviously malignant. The latter is the case in this patient. It may present as a non-healing ulcer, or as red, white or mixed red-and-white lesions. Characteristic signs of oral SCC are non-healing ulcer, ulcer with rolled borders, fungation (this patient), fixation and induration. Rarely, oral SCC may present as unexplained, asymptomatic lateral neck lymphadenopathy (1-3). The location, age, risk factors and clinical presentation of this case are typical of oral SCC. The histology of this specimen was that of a poorly differentiated round cell neoplasm, but the immunohistochemistry markers were clearly not epithelial in nature.
Sorry! you are incorrect
Given the patient’s history of kidney cancer, tumor metastasis has to be considered very seriously in this case. Cancer metastasis to the oral cavity is neither specific nor common. Although it constitutes less than 1% of all oral malignant neoplasms, it may have a devastating result to the patient mainly because metastasis to other sites has already developed or is inevitable (4-5). Theoretically, any malignant neoplasm can metastasize to the oral cavity, but in actuality few do and out of the ones that do, the majority are carcinomas rather than sarcomas. The most common malignant neoplasms that metastasize to the mouth are from the breast, lung, kidney and prostate (4-5). Malignant neoplasms from the thyroid, pancreas, colon, and liver have also been described. Breast cancer is the most common neoplasm to metastasize to the oral cavity regardless of gender. However, lung and prostate cancers are the most common neoplasms to metastasize to the oral cavity in men. In the most cases, the oral presentation is a secondary diagnosis when the primary diagnosis of the distant organ has been already made and the patient has had or is undergoing treatment for it. This was true of our patient. Although rare, it is known that on occasion the oral lesion is the first manifestation of the disease. By far the most common location is the posterior mandible, where 80% of cases occur, followed by the gingiva. The maxilla is a rare location for tumor metastasis. It is typically described in adults over the age of 30 and rarely in children. Pain and swelling are the most common clinical symptoms, which was the case with this patient. It may also present as asymptomatic, simulating a periapical lesion or it can cause anesthesia and parasthesia, especially when it involves the inferior alveolar canal. The latter results in so-called “numb-chin syndrome.” Tooth loosening, displacement and sharp resorption have also been described. Gingival swelling, like a pyogenic granuloma, has also been described which how this patient presented. The radiographic appearance of irregular bony destruction is also common for metastatic tumor. The majority of neoplasms cause bony destruction with ill-defined borders, the moth-eaten appearance of some bony destruction indicating aggressive behavior. It is also important to mention that at times, well-demarcated lesions with a benign morphology, as well as cystic radiographic morphology, have also been described. Metastatic neoplasms from the prostate may also be bone-forming, resulting in either radiopaque or mixed radiopaque and radiolucent lesions misdiagnosed as a benign fibro-osseous lesion.
As in this case, the diagnosis of tumor metastasis to the oral cavity carries a poor prognosis because the oral cavity is usually not an isolated site and tends to project more disseminated clinical behavior (4-5). Patients are typically treated with chemotherapy and the five-year survival rate is very low. The histology was not supportive of a metastatic kidney cancer.
Sorry! you are incorrect
Pyogenic granuloma constitutes 85% of all reactive gingival swellings, representing a profuse mass of vascular granulation tissue (6). It can be induced by local irritants such as excessive plaque, sharp fillings and dental calculus; it sometimes forms in an extraction socket in response to an irritant left in the socket. It can occur anywhere in the oral cavity and skin, especially the tongue, lips, fingers and nail beds (6). In the mouth, it occurs most commonly in the gingiva, especially the maxillary buccal and interproximal gingiva (7). Occasionally, it may surround the tooth. It is usually highly vascular, fast-growing, exophytic, lobular, sessile, and ulcerated or covered by ppseudomembrane. The color changes from red to pink when it starts to heal. It occurs at any age and sex with a slight predilection for young females; it affects 1% of pregnant females. Pyogenic granuloma is usually painless except during eating, when bleeding and pain are described (6). Histologically, it presents as a mass of loose and vascular granulation tissue, usually with ulcerated or eroded surface epithelium and many inflammatory cells. A range of treatment modalities are available, including excision with removal of the local irritant, laser surgery, or intralesional injection with absolute alcohol, steroids or botulinum toxin (7-8). Scaling and polishing prior to surgical removal helps shrink the lesion. The prognosis is good, although recurrence is possible, especially during pregnancy. The clinical presentation is suggestive of pyogenic granuloma; the histology, however, is not.
Sorry! you are incorrect
Peripheral giant cell granuloma constitutes less than 5% of all reactive gingival swellings, and consists of a hyperplastic mass of vascular granulation tissue with many osteoclast-like multinucleated giant cells. It presents as a lobular, purplish-blue exophytic nodule exclusively on the gingiva, both edentulous and dentate, and usually anterior to the molars (9-10). It originates from either the periodontal ligament or the periosteum. It occurs across a wide age range, especially in children, young adults, and females (2:1 female to male ratio) (9-11). It presents as either sessile or pedunculated and smooth surfaced or lobular. Though usually painless, it can occasionally be ulcerated, painful and accompanied by bleeding (9-11). Like pyogenic granuloma, it is usually present either on the buccal or lingual gingiva or between teeth, but it can occasionally surround the teeth (9-11) and act aggressively by displacing teeth much like a sarcoma (9). It can also resorb the underlying bone in a smooth and concave “saucer-like” manner. Complete excision including curettage of underlying bone is the preferred treatment. It has a good prognosis with recurrence rate of approximately 10% (11). The clinical presentation is suggestive of peripheral giant cell granuloma; the histology however, is not.
Congratulations! You are correct
Post transplant lymphoproliferative disorder (PTLD) is a serious disease associated with organ transplant patients as a result of significant immunosuppression induced by medications such as Cyclosporine and in this case Sirolimus . It is result of uncontrolled proliferation of B lymphocytes induced by Epstein Barr Virus infection. The uncontrolled proliferation may have a malignant potential as is the case in this patient.
The use of transplantation to replace diseased or failing organs is now a widely accepted modality of therapy which includes lung, heart, kidney, liver, bone marrow and at times, combination of organs (12, 13). This patient was the recipient of bilateral lungs for chronic obstructive pulmonary disease (COPD). One-and 5-year survival rates for lung transplantation are about 70% and 40%, respectively (14). In large measure, this has been made possible by the introduction of more potent immunosuppressive regimens such as the use of cyclosporine, Sirolimus and Tacrolimus (13). Today in the USA, almost 30,000 cases of organ transplant are performed, many of which will experience complications, with some very serious such as the PTLD. This potentially fatal disease is the result of chronic immune suppression; it is particularly associated with solid organ transplant patients such as lung, heart, liver, intestine and sometimes with kidney transplants. Excluding squamous cell carcinomas of the skin and other malignancies of the skin, PTLD is the most common complication of organ transplant. Reduction and/or withdrawal of the immunosuppressive agents can be a treatment for PTLD but that would carry the serious risk of rejection of the organ and be fatal for the patient.
The most common post-transplant oral changes include gingival hyperplasia described in cyclosporine and Sirolimus patients (15). PTLD has been described in the mouth but rarely and is most commonly described with cyclosporine. This communication presents PTLD of the mouth in association with Sirolimus, which may be the first of its kind. PTLD more commonly occurs outside the mouth; it occurs in a variety of locations, as described by Penn and First (16).
PTLD represents a spectrum of diseases ranging from benign proliferation of inflammatory and mesenchymal cells such as reactive lymphoid hyperplasia to aggressive malignant non-Hodgkin’s lymphomas. The development of malignant lymphomas in patients who had received transplants was described by Penn et al. (15-17). A tumor registry kept by Penn (16-17) shows that the overall incidence of cancers in transplant patients averages 6%, with a range of 1-18%. These cancers show an elevated proportion of lymphomas (20% vs. 5% of cancers in the general population), of which 94% are non-Hodgkin’s lymphoma (vs. 65% in the general population). Of these, 86% are of B cell origin, 13% are of T cell, and 1% are of null cell origin. A marked difference is seen when lymphoma incidence is observed in both transplant patients treated with cyclosporine (± prednisone) and those not treated with cyclosporine (azathioprine or cyclophosphamide with prednisone). Twenty-seven percent of cancers in the cyclosporine patients are lymphomas, compared with 12% in the noncyclosporine group (17).It has been shown that PTLD is almost invariably associated with a primary infection by EBV. For example, Berg et al. (19) used in situ hybridization with a probe for the BamH1-W region of the EBV genome to study 52 specimens from 28 patients. EBV was identified in 26/28 patients, and the other 2 showed evidence of EBV by filter hybridization. EBV induces uncontrolled proliferation of B cells in immunodeficient patients (12-16) and, with the use of powerful immunosuppressive drugs there is inadequate T cell control over EBV-driven proliferation of the infected B cells (18).
In terms of risk factors, host immunosuppression is the chief predisposing factor for PTLD. Some studies report the relative incidence to be 1% for renal transplants, 1.8% for cardiac, 2.2% for liver and 4.6% for heart-lung (12-18). Heart-lung recipients are predisposed to acute rejection episodes and opportunistic infections; PTLD occurred in 9.4% of these patients in one study from Pittsburgh (15). It was suggested that cyclosporine in combination with other immunosuppressants increased the likelihood of the development of lymphomas and that the use of cyclosporine alone might decrease the tendency for lymphomas to develop. An increased incidence of PTLD has been reported in lung and cardiac transplant recipients (16). The exact etiology of this is not clear, but is suggested to be related to increased immunosuppression (taking higher doses of immunosuppressive agents) and an associated increase in the incidence of viral infection (15-16). A longitudinal study showed that, over a 6-month period, there was a significant correlation between mean plasma concentration of cyclosporine and increase in gingival hyperplasia, one of the symptoms of PTLD (15).
The clinical presentation of PTLD can vary from a self-limiting mononucleosis to diffuse polyclonal, from oligoclonal lymphoid proliferation to lymphoma, or from nonclonal hyperplasias to monoclonal or ligoclonal tumors. Lymphoproliferative disorders in association with transplants are unique, with a predilection for extranodal sites as well as the association with EBV. The primary occurrence is typically at the site of the transplanted organ; for example, in 60% of heart-lung transplant patients, the disease occurred primarily in the grafted lungs and was associated with a primary infection by EBV. There is frequent absence of immunophenotypic and genotypic evidence of monoclonality and poor response to cytolytic or radiation therapy regimens used for treatment of lymphoma. The EBV-positive monomorphic form of PTLD often shows a poor response to reduction in immunosuppressive therapy (15).
PTLD may occur months or even years after organ transplantation, so it may be seen first in institutions that are not involved in transplantation (15). It is essential that the lymphoproliferative syndrome be recognized and treated early, as many cases can be cured. Without accurate diagnosis and prompt treatment, rapid fatality is a potential. The prognosis is particularly poor in bone marrow recipients; in one study, 29/32 patients died. PTLD also is unique in regard to its potential for resolution by restitution of immune competency, except in the case of the monomorphic EBV-positive type. Overall, about 40% of patients with the syndrome survive, either by spontaneous remission on withdrawing the immunosuppressive agent or because of the efficacy of acyclovir, ganciclovir, or radiotherapy in the patients with lymphoma (15).
Treatment
An incisional biopsy was performed under local anesthesia. The patient was referred to his physician for further treatment of the PTLD.
References
- Examinations for oral cancer – United States. MMWR. Morb.MortalWkly Rep. 1992; 43:198.
- Barasch A, DE Morse, et al. Smoking, gender, and age as risk factors for site-specific intraoral squamous cell carcinoma. Cancer 1994; 73:509-513.
- American Joint Committee for Cancer Staging and End Result Reporting. 1983.
- A. Hirshberg and A. Buchner , Metastatic tumours to the oral region. An overview. Oral Oncol. 1995; 31: 355–360.
- van der Waal, RIF, Buter, J. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg. 2003; 41: 3-6.
- Fantasia JE, Damm DD. Red nodular lesion of tongue. Pyogenic granuloma. Gen Dent. 2003 Mar-Apr;51(2):190, 194.
- Ichimiya M, Yoshikawa Y, Hamamoto Y, Muto M. Successful treatment of pyogenic granuloma with injection of absolute ethanol. J Dermatol. 2004 Apr;31(4):342-4.
- Pham J, Yin S, Morgan M, Stucker F, Nathan CA. Botulinum toxin: helpful adjunct to early resolution of laryngeal granulomas. J Laryngol Otol. 2004 Oct;118(10):781-5.
- Flaitz CM, Peripheral giant cell granuloma: a potentially aggressive lesion in children.Pediatr Dent. 2000 May-Jun;22(3):232-3.
- Chaparro-Avendano AV, Berini-Aytes L, Gay-Escoda C. Peripheral giant cell granuloma. A report of five cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2005 Jan-Feb;10(1):53-7; 48-52.
- Neville BW, Damm DD, Allen CM, Bouquot JE. Peripheral giant cell granuloma. In: Oral and Maxillofacial Pathology, 2nd edition. Philadelphia: W.B. Saunders, 2002. p. 449-451.
- Antin JK, Kim H, Cutler C et al. Sirolimus, Tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood 2003; 102:1601.
- Cutler, C, Kim, H, Hochberg, E, et al. Sirolimus and Tacrolimus Without Methotrexate as Graft-Versus-Host Disease Prophylaxis After Matched Related Donor Peripheral Blood Stem Cell Transplantation. Biol Blood Marrow Transplant 2004; 10:328.
- Kaye MP. The Registry of the International Society for Heart and Lung Transplantation: tenth official report-1993. J Heart Lung Transplant 1993; 12: 541
- Oda, D., Persson, R., Sabbath, D., Haigh, W.G., Penn, I., and Aziz, S. Oral presentation of post-transplantation lymphoproliferative disorder: an unusual manifestation. Transplantation. 61:435-440, 1996.
- Penn I, First MR. Development and incidence of cancer following cyclosporine therapy. Transplant Proc 1986; 18 (suppl 1): 210.
- Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc 1969; 1: 106.
- Matas AJ, Hertel BF, Rosai J, Simmons RL, Najarian JS. Post-transplant malignant lymphoma: distinctive morphologic features related to its pathogenesis. Am J Med 1976; 61: 716.
- Berg LC, Copenhaver CM, Morrison VA, et al. B cell lymphopro-liferative disorders in solid-organ transplant patients: detection of Epstein-Barr virus by in situ hybridization. Hum Pathol 1992; 23: 159.