Well-Demarcated, Locally Destructive Radiolucency, Right Posterior Mandible
Can you make the correct diagnosis?
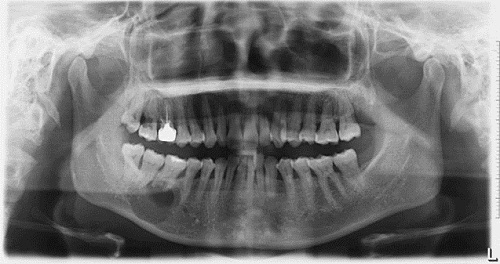
This is a 39-year-old Asian female who presented with an expansile and well-demarcated radiolucency involving teeth #s 30 & 32.
Sorry! you are incorrect
The age of the patient, the location and apparent perforation of the bone is all in support of odontogenic keratocyst (OKC) and for that reason, should be considered and placed high on the differential diagnosis list. However the described expansion of the bone is unlike the behavior of an OKC that hollows rather than expands bone, neither is the lack of tooth vitality. The histology is not supportive of an OKC.
Odontogenic keratocyst is an aggressive cyst known for its rapid growth and its tendency to invade the adjacent tissues, including bone. It has a high recurrence rate and is associated with bifid rib basal cell nevus syndrome. The majority of patients are in the age ranges of 20-29 and 40-59, but cases in patients ranging in age from 5 to 80 years have been reported. The distribution between sexes varies from equal distribution to a male-to-female ratio of 1.6:1, except in children. Odontogenic keratocyst predominantly affects Caucasian populations and, if one may judge from the limited evidence provided by the literature, is chiefly of Northern European descent.
Odontogenic keratocysts may occur in any part of the upper and lower jaw, with the majority (almost 70%) occurring in the mandible. They occur most commonly in the angle of the mandible and ramus. Posterior mandible is an area common to many benign odontogenic tumors such as ameloblastoma and odontogenic myxoma and is also a typical location for dentigerous cysts. Radiographically, OKCs present predominantly as unilocular radiolucencies with well-defined, sclerotic or scalloped borders. They may also present as multilocular radiolucencies. Odontogenic keratocysts of the maxilla are smaller in size when compared to those occurring in the mandible; larger OKCs tend to expand bone, but mildly–obvious clinical expansion (which is the case in this patient) should be viewed with suspicion for a neoplasm. OKCs can also present as small and oval radiolucencies between teeth simulating a lateral periodontal cyst, in an area of an extracted tooth simulating a residual cyst, at the apex of a vital tooth mistaken for a periapical cyst, or in the anterior maxilla between the central incisors simulating an incisive canal cyst. OKCs grow to sizes larger than any other odontogenic cysts. They usually penetrate the bone rather than expand it and grow in an anterior to posterior direction. Despite this aggressive growth, they often remain asymptomatic, thus growing to large sizes and hollowing the bone.
Odontogenic keratocysts are significant clinical entities due to their tendency for recurrence and destructive behavior. They are known to have a high recurrence rate, ranging from 13% to 60% (1, 2). Complete surgical removal is the treatment of choice. Surgery includes enucleation, curettage, enucleation and peripheral ostectomy, and resection depending on the radiographic presentation, location and clinical behavior. Surgery combined with Carnoy’s solution or liquid nitrogen treatment has been effective in reducing recurrence rate. At times, adjacent or associated teeth are extracted in the interest of complete removal. Some investigators advocate marsupialization and occasionally resection of the more aggressive cysts that tend to perforate buccal and lingual bone. Resection is a rare modality of treatment. Most cysts recur within the first three years while others may recur as late as after 16 years. Conservative surgical removal and long-term follow-up is the treatment of choice by most clinicians.
Sorry! you are incorrect
The age of the patient, the expansion and perforation of bone are all in support of the behavior of a neoplasm including a neoplasm of tooth origin such as an odontogenic myxoma. The lack of tooth vitality is not supportive of an odontogenic myxoma. Nonetheless, odontogenic myxoma should be placed high on the differential diagnosis. The histology however, is not supportive of an odontogenic myxoma.
Odontogenic myxoma occurs in the jaw bones, usually in the tooth-bearing areas of the jaw. It is an uncommon, benign, but locally aggressive neoplasm. Nearly all cases so far have been described in the jaw bones. Therefore, it is of tooth origin, and is believed to be from the mesenchymal portion of a tooth germ, most likely of the dental papilla. It has the potential for extensive bony destruction and extension into the surrounding structures. It is less common than odontomas and ameloblastomas. For that reason, a pathologist who is not familiar with the histology of a tooth germ can mistake a myxoid dental follicle for an odontogenic myxoma. Almost 75% of odontogenic myxomas occur in patients around 23-30 years of age with a slight female predilection (1:1.5 male-to-female). It rarely occurs in patients over 50 or under 10 years of age. It occurs almost equally in the maxilla and mandible with a slight predilection for the posterior mandible. A few cases are described in the ramus and condyle, non-tooth bearing areas.
Odontogenic myxoma is slow-growing, persistent and destructive. Most cases are expansile and can displace and resorb teeth. In the maxilla, they usually invade the maxillary sinuses and at times (though rarely) cross the midline to the opposing sinus. Radiographically, the majority present as expansile and multilocular, though some are unilocular with or without scalloped borders, and rare cases present with a diffuse and mottled appearance which can be mistaken for a malignant neoplasm. Grossly, this lesion is gelatinous in nature, making curettage alone difficult; the more fibrotic odontogenic myxomas (also known as odontogenic myxofibroma or fibromyxoma) have more body and are easier to curette. Histologically, it is made up of loose and delicate fibrous connective tissue. The fibroblasts are stellate and are suspended on a delicate network of collagen fibrils. Immunohistochemistry studies suggest that the spindle-shaped cells constituting this neoplasm have a combined fibroblastic and smooth muscle typing, suggesting that it is of myofibroblastic origin. Small blood vessels are present, as are small odontogenic epithelial islands on occasion. Sometimes, this lesion is fibrotic, making it easier to curette.
The treatment of choice is surgical excision ranging from segmental resection with clear bony margins of up 1.5cm to prevent recurrence of the neoplasm. Curettage is with and without cauterization is used for treatment, but is associated with a high recurrence rate. Reconstruction can be immediate or delayed, and can include an autologous bone graft from the anterior or posterior iliac crest. Fibula-free vascular osteocutaneous bone graft is another reconstructive modality, as is distraction osteogenesis. Immediate postoperative follow up is weekly for approximately one month, then monthly for the next five months and twice a year for the next five years.
Sorry! you are incorrect
The expansion and the location are a good presentation for ameloblastomas in general. Ameloblastomas are a family of neoplasms with varied clinical and radiographic presentation. For example, unicystic ameloblastoma would be the more likely diagnosis in this case if the unilocular radiolucency is taken into account as the most significant finding. However, neither the age, nor the perforation of the jaw bone is supportive of unicystic ameloblastoma. On the other hand the age and bony perforation is a good presentation for the conventional (solid, multicystic) ameloblastoma. The latter tends to be radiographically multilocular rather than unilocular. In addition, 90% of unicystic ameloblastomas occur in association with an impacted tooth resembling a dentigerous cyst. The histology in this case was not supportive of any type of ameloblastoma.
Ameloblastoma is one of the most common benign neoplasms of odontogenic origin. It accounts for 11% of all odontogenic neoplasms. It is a slow-growing, persistent, and locally aggressive neoplasm of epithelial origin. It affects a wide range of age distribution but is mostly a disease of adults, at an average age of 33, with equal sex distribution. Reports from Africa and India show a male predilection; it also has a predilection for occurrence in black patients. The location and age of this patient can be consistent with an ameloblastoma. The unilocular radiolucency is unusual for the solid type but is consistent with the 10% of the unicystic ameloblastomas that occur in an extra-follicular manner. About 85% of ameloblastomas occur in the posterior mandible; most of these occur in the molar-ramus area, and some occur in the anterior mandible. Three types are described including solid ameloblastoma which is characteristically expansile, radiolucent and multilocular and not consistent with this case. The unicystic type is radiographically unilocular but in 90% of the time is associated with the crown of an impacted tooth. The other 10% are unilocular radiolucency usually associated with teeth such as between teeth. The patients constituting this group are much younger in age and are around 14-20 years of age. The third type occurs on the gingival without bony involvement and is known as peripheral ameloblastoma. Clinically, it resembles a reactive gingival swelling such as pyogenic granuloma or peripheral ossifying fibroma. It is rare and accounts for less than 1% of all ameloblastomas.
Ameloblastoma, if not treated, can reach very large sizes, causing facial disfigurement. It loosens, displaces and resorbs adjacent teeth. Ameloblastomas are usually not painful unless infected, in which case they can be mildly painful. Parasthesia and anesthesia are extremely rare, unless the lesion is very large in size. Also, ameloblastoma tends to expand rather than perforate the cortical bone; if the latter occurs with extension into the adjacent soft tissue, it has a higher tendency for recurrence and therefore a worse prognosis than cases in which the ameloblastoma is completely encased by bone. The solid type is treated with en bloc or resection with clean margins. Curettage is the treatment of choice for the unicystic type.
Congratulations! You are correct
The age, the gender, location and the bony expansion are all in support of a central ossifying fibroma diagnosis. The perforation of the lingual plate is unlike the clinical behavior of this condition neither is the lack of vitality of tooth #30. The latter can be explained by the presence of a periapical cyst at the apex of tooth #30 which explains the lack of vitality and also sheds light on the etiology being a tooth related rather than neoplasm related thus identifying two pathologic conditions existing in this one area. The two pathologies are separate and unrelated.
Central ossifying fibroma is a benign neoplasm of bone origin. It presents as a well-demarcated to corticated radiolucent or mixed radiolucent/radiopaque mass with a peripheral radiolucent rim. Central ossifying fibroma is a slow-growing, expansile lesion with characteristic downward expansion of the inferior border of the mandible. It can also expand buccally and lingually. The associated teeth are vital. It is common in young adults around 35 years of age and is five times more likely to occur in females than males. It affects the posterior mandible in about 90% of cases. Curettage is the treatment of choice and recurrence is rare.
References
- Shear M. Odontogenic keratocysts: natural history and immunohistochemistry. Oral Maxillofacial Surg Clin N Am. 2003; 15: 347-362.
- Oda D, Rivera V et al. Odontogenic keratocyst: the northwestern USA experience. J Contemp Dent Pract. 2000 Feb 15; 1(2): 60-74.
- Zachariades N, Papanicolaou S et al. Odontogenic keratocysts: Review of the literature and report of sixteen cases. J Oral Maxillofac Surg. 1985; 43: 177-182.
- Reichart PA, Philipsen HP. et al. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol 1995;31B:86–99.
- Julio César Bisinelli, Sérgio Ioshii, Luciana Borges Retamoso, Simone Tetü Moysés, Samuel Jorge Moysés and Orlando Motohiro Tanaka. Conservative treatment of unicystic ameloblastoma. American Journal of Orthodontics and Dentofacial Orthopedics. Volume 137, Issue 3, March 2010, Pages 396-400.
- Gardner DG. Some current concepts on the pathology of ameloblastomas. J Oral Maxillofac Surg 1996; 82:660-669.
- Sharma R, Marwah N. Odontogenic myxoma of the mandible: a case report. Indian J Pathol Microbiol. 2003;46:84-86.
- Simon EN, Merkx MA. Odontogenic myxoma: a clinicopathological study of 33 cases. Int J Oral Maxillofac Surg. 2004;33:333-337.
- Moshiri, S., Oda, D.et al. Odontogenic myxoma: a clinical and immunohistochemical study. J Oral Path 1992;21:401-403.
- Su L, Weathers DR, et al. Distinguishing features of focal cemento-osseous dysplasia and cemento-ossifying fibromas: II. A clinical and radiologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997 Nov; 84: 540-549.