Small flat dark brown lesion right anterior floor of mouth
Can you make the correct diagnosis?
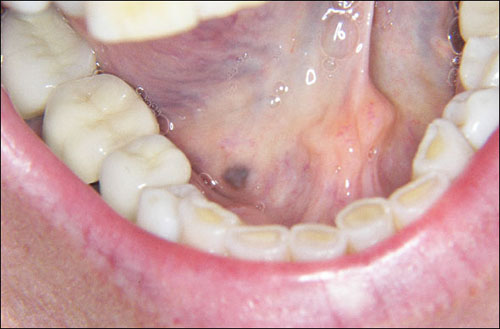
This is a 53-year-old white female referred by her dentist for the evaluation of a 4 x 3 mm flat, well-demarcated pigmented lesion on the right anterior floor of mouth.
Sorry! you are incorrect
The floor of the mouth is a very uncommon location for pigmented lesions. Nonetheless, given that this lesion is focally dark brown to black and focally blue in color, one should consider a pigmented nevus on the differential diagnosis with the understanding that pigmented nevi are exceedingly rare in the floor of the mouth. The histology is not supportive of a melanocytic nevus. Melanocytic nevi (common moles) are rare in the mouth but are very common on the skin. Most skin nevi are the acquired (developmental) type while congenital nevi are much less frequent on the skin and also far less frequent in the mouth. Nevi are benign and most likely hamartomatous proliferations of cells of neural crest origin which can be melanocytes or other close related cells. In the oral cavity, they most commonly occur on the hard palate, buccal mucosa, gingiva and lips. They are more common in females around 30 years of age. Intramucosal (intradermal) and blue nevi constitute 80-90% of all oral melanocytic nevi with compound and junctional nevi accounting for less than 10-20% of cases. Nevi vary in color, from colorless to dark blue, brown or black; some oral nevi lack the brown color. They can be flat, raised, smooth-surfaced or papillary with a smooth border and symmetrical morphology. Histologically, they vary in morphology depending on the type. Nevi are usually not removed unless they are chronically irritated. They are sometimes removed for aesthetic reasons. They should be removed if they change in appearance in accordance with the “A-E rule”: A) for asymmetry, B) for border (irregular border), C) for color (lack of uniformity in color), D) for diameter (larger than six mm in diameter) and E) for enlarging. They should be surgically removed if they ulcerate or bleed.
Congratulations! You are correct
Neither the floor of the mouth nor the brown color, are supportive of amalgam tattoo’s clinical presentation. However the lesion is focally blue to black in color would be consistent with amalgam tattoo. Amalgam tattoo tends to occur near teeth filled with amalgam such as on the gingiva and the alveolar mucosa. It tends to be flat, blue and irregular. Despite the unusual location, the histology of this specimen was that of an amalgam tattoo and thus it is the rendered diagnosis.
Amalgam tattoo is only second to racial pigmentation in being the most common pigmented lesion in the oral cavity. It is an example of a local deposition of exogenous metallic material from a dental filling, usually the silver metal found in an amalgam. The fine dark granules of this material are deposited on the very thin collagen fibers of the connective tissue, around small blood vessels, on the basement membrane, and between muscle fibers, salivary gland tissue and fat. Amalgam tattoo occurs as a result of metal implantation in lacerated mucosa during a dental filling or the removal of an old amalgam filling. It can also be the result of amalgam falling in the dental socket following an extraction or in the area of an apicoectomy sealing. Like any cutaneous tattoo, it becomes part of the live tissue and leads to a permanent coloration of the mucosa, usually flat and grey to bluish-black or black in color and rarely, as is the case in this patient, dark brown in color. Occasionally, it can be exophytic or nodular; this is usually the result of a foreign body reaction to the metal. The margins can be irregular, well defined or diffuse, blending in with the surrounding unaffected mucosa. The borders are usually flat and not rolled. They can enlarge and give the impression of lateral spread; this happens during the first months of implantation, especially when a foreign body reaction with macrophages presents, carrying the material laterally. This may make the clinician think beyond amalgam tattoo to a more sinister disease. Once the area scars, the lateral spread ceases. Amalgam tattoo most commonly occurs on the alveolar mucosa, buccal mucosa, and gingiva, but has been described on the palate, floor of mouth and tongue. It can be viewed radiographically, especially within a socket if present in clusters. Histologically, it presents as granular black material deposited on the surrounding thin collagen fibrils. Sometimes, foreign body reaction with foreign-body-type giant cells, chronic inflammation and scarring are present. Sometimes, an incisional biopsy is recommended to eliminate the possibility of other, more serious diseases; otherwise, there is no need for treatment.
References
- Meleti M, Vescovi P, Mooi WJ, van der Waal I. Pigmented lesions of the oral mucosa and perioral tissues: a flow-chart for the diagnosis and some recommendations for the management. Oral Surg Oral Med Oral Pathol Oral Radiol. 2008 May;105:606-16.
- Torres Fernández G. Pigmented lesion of the oral cavity with eight years follow-up. P R Health Sci J. 2000 Jun;19(2):165-8.
- Buchner, P.W. Merrel and W.M. Carpenter, Relative frequency of solitary melanocytic lesions of the oral mucosa, J Oral Pathol Med 33 (2004), pp. 550-557.
- Eisen, Disorders of pigmentation in the oral cavity, Clin Dermatol 18 (2000), pp. 579-587.
- Çiçek and Ü. Ertas, The normal and pathological pigmentation of oral mucous membrane: a review, J Contemp Dent Pract 3 (2003), pp. 76-86.
- Healsmith, MF, Bourke, JF et al. An evaluation of the revised seven-point check list for the early diagnosis of cutaneous malignant melanoma. Br J Dermatol 1994; 130: 48-50.
- Owens BM, Johnson WW, Schuman NJ. Oral amalgam pigmentations (tattoos): a retrospective study. Quintessence Int. 1992 Dec;23(12):805-10.
- Shah, G, Alster, TS. Treatment of an amalgam tattoo with a Q-switched alexandrite (755 nm) laser. Dermatol Surg 2002; 28: 1180-1181.
- Amano H, Tamura A, Yasuda M, Yamanaka M, Takeuchi Y, Sasaoka K, Yokoo S, Ishikawa O. Amalgam tattoo of the oral mucosa mimics malignant melanoma. Dermatol. 2011 Jan;38(1):101-3.
- Pigatto PD, Brambilla L, Guzzi G. Amalgam tattoo: a close-up view. J Eur Acad Dermatol Venereol 2006; 20: 1352-1353.
- Jainkittivong A, Aneksuk, V, and Langlais RP. Oral mucosal conditions in elderly dental patients. Oral diseases.2002; 8: 218-223.
- Moreira RN, Santos CR, Lima NL, Verli FD, Marinho SA. Oral and cutaneous melanoma: similarities and differences. Clin Med Res. 2010 Aug 18;2(4):155-8.
- Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000 Mar; 36(2):152-169.
- Rapidis, AD, Apostolidis, C et al. Primary malignant melanoma of the oral mucosa. J Oral Maxillofac Surg. 2003; 61: 1132-1139.
- Rigel, DS, Friedman, RJ et al. The incidence of malignant Melanoma in the United States: issue as we approach the 21st century. Am J Acad Dermatol 1996; 34: 839-847.
Sorry! you are incorrect
As stated previously, the floor of the mouth is a very uncommon location for pigmented lesions. However, since this lesion is focally dark brown in color, one should consider oral melanotic macule on the differential diagnosis. The location is not supportive of a melanotic macule; neither is the histology.
Melanotic macules of the mouth are more common than melanocytic nevi. They present as oval-shaped brown, smooth bordered and flat lesions less than 8 mm in diameter, mostly on the lower lip near the midline. Melanotic macules of the lip are called labial melanotic macules and the ones that occur in the oral cavity are called oral melanotic macules. Oral melanotic macules are more common on the gingiva, followed by the buccal mucosa. Oral melanotic macules can occur at any age, with a 2:1 prevalence in females. They usually present as a solitary lesion, but can present in multiples. When multiple macules are identified, it is necessary to rule out Adrenal insufficiency, Peutz-Jeghers syndrome, Laugier-Hunziker syndrome; smoking-associated hyperpigmentation, and drug-induced hyperpigmentation.
Sorry! you are incorrect
The lack of uniformity in the color should make one think beyond benign pigmented lesions and consider oral malignant melanoma, though with the understanding that primary malignant melanoma of the mouth is exceedingly rare. The location is highly unusual for malignant melanoma. The histology is not supportive of malignant melanoma. Malignant melanoma (MM), whether on the skin or in the oral cavity, has the same cell of origin: the melanocyte. Despite the common cell origin, however, these lesions have different clinical behavior. Mucosal melanomas are very rare and are more aggressive than those of the skin. They usually affect adults with an average age of 55; occasionally, they affect patients under 30 years of age. Skin MM used to have a poor prognosis but that has changed significantly; in 1950, the 5-year survival rate for skin MM was 50%, while today it is 90%. However, mucosal melanomas still carry a dismal 5-year survival rate of not higher than 38%. Skin MM, like mucosal melanoma, occurs mostly in adults around 50 years of age and rarely affects children. Cutaneous malignant melanoma is the third most common skin epithelial malignancy (following basal cell carcinoma and squamous cell carcinoma) but by far the most aggressive of the three. In the United States alone, over 50,000 cases are diagnosed annually with about 8,000 dying of the disease each year. It has been on the increase since 1960 and has recently plateaued. In the 1960s, 1 in 600 individuals were expected to develop MM in their lifetime, compared with 1 in 71 in 2001. Australia has the highest number of skin MMs, a number that has been doubling every 10 years. The etiology of MM and its rate of increase are complex and are beyond the scope of this discussion. However, it is important to mention that multiple factors seem to play a role the development of MM and its progression. The most important factor is exposure to ultraviolet light, which explains cases on the skin but does not explain the oral, nasal, and other non-sun exposed areas that develop MM. It has been suggested that the age of the individual at the time of exposure is important. It is also suggested that sunburns, especially blistering sunburns in childhood, increase the risk of developing MM in adulthood. Genetics play a significant role in MM, especially in patients born in families with a history of this disease. For instance, patients from families with a history of dysplastic mole syndrome or patients who have a first relative with malignant melanoma are at a higher risk of developing the disease. 70% of cutaneous MMs occur in a preexisting pigmented nevus. Pigmented nevi are common and have a one in a million chance of transformation to MM. This does not necessitate surgical removal, but rather suggests that practitioners should be attentive to alterations in nevi such as changes in color, shape and size; ulceration and/or bleeding; inflammation; pain; and a diameter change of greater than 7mm. These are the clinical criteria used in England to assess the clinical presentation of MM. Bleeding and ulceration of a pigmented nevus should be viewed seriously, and the lesion should be soon biopsied or completely removed and submitted for microscopic evaluation. The clinical features of MM in the United States follow the ABCDE rule: asymmetry, borders, color, diameter and enlargement. A pigmented lesion that is asymmetrical with irregular borders (especially borders with notching) and uneven coloration (a range of tan to dark brown as is the case with our patient), is larger than 6 mm in diameter, and continues to enlarge should be viewed with suspicion and biopsied immediately. The oral mucosa is rarely affected by MM and for that reason, the etiology of oral malignant melanoma is still unclear. Local melanosis and tobacco use have been implicated. Race also appears to play a role; for example, 7.5% of all reported oral melanomas affect those of Japanese descent and 10% affect those of Ugandan descent, compared with less than 1% of cases occurring in Caucasians. Cutaneous MM, however, occurs at a much higher rate in Caucasians than in those with brown or black skin, with a rate of 1:83 in whites compared to 1:1176 in dark-skinned individuals. Mucosal melanomas are extremely rare compared to cutaneous melanomas; they constitute less than 1% of all MMs. For that reason, it is always prudent to first rule out metastatic MM to the oral cavity from the skin when a biopsy of an oral pigmented lesion confirms the diagnosis of MM. 80% of oral MMs affect the palate and the maxillary gingiva. Mandibular gingiva can be affected, but rarely. Cutaneous MMs occur mostly on the interscapular area of the back and back of the legs. 25% occur in the head and neck area, including the face, eyes, nose and mouth. In the oral cavity, they present as flat, multiple, graded or with uneven coloration, and irregular borders; they are sometimes ulcerated. They are usually asymptomatic; pain is described only rarely. Histologically, MMs demonstrate radial and vertical growth patterns. The vertical pattern is important because it plays an important role in predicting prognosis; for that reason, the depth of invasion is always reported. Depth of invasion in skin MMs is fully established following Clark and Breslow’s staging according to depth. Obviously, the deeper the tumor invasion, the higher the microscopic staging and the worse the clinical behavior. Determining prognosis is not simple, though; other factors play a role in worsening the prognosis, such as the location, age and gender of the patient. Surgery is the treatment of choice treatment for MM of the oral cavity. Radiation and chemotherapy may be used in advanced cases.