Fosamax induced osteonecrosis
Can you make the correct diagnosis?
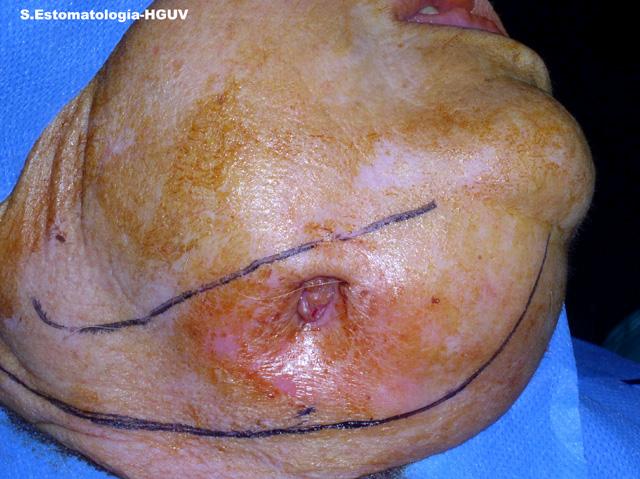
This is an 82-year-old white female from Spain diagnosed with osteoporosis a few years prior. She was treated with Fosamax at a dosage of 70 mg per week for three years.
Sorry! you are incorrect
It is uncommon for osteosarcoma to cause jaw bone suppuration and cutaneous fistula. The latter is more commonly seen with periapical abscesses breaking through the bone into the soft tissue in form of osteomyelitis. Osteosarcoma is the most common, non-hematopoietic, primary malignancy of bone. It is a malignancy of mesenchymal cells that have the ability to produce osteoid or immature bone. Osteosarcoma of the jaw represents 6-9% of all osteosarcomas. Paget’s disease and prior radiation therapy are associated with an increased risk of developing osteosarcoma. The mean age for patients with jaw osteosarcomas is about 33 years, which is 10-15 years older than the mean age of osteosarcomas of the long bones (3-5). The maxilla and mandible are involved with equal frequency. Patients with osteosarcoma may experience pain, swelling, parasthesia and/or loosening of teeth (5). These tumors may vary greatly in radiographic presentation. Some lesions display an entirely radiolucent process, while others may demonstrate dense sclerosis in the affected area. However, the majority of osteosarcoma cases present as a mixed radiolucent and radiopaque lesion. Other radiographic findings include: symmetric widening of the periodontal ligament space (PDL), diffuse borders of the lesion, periosteal reaction, “spiked” roots or the classic “sunburst” or “sun ray” appearance caused by osteophytic bone deposition at the periphery (3-5). These changes may be evident on CT, periapical, panoramic, and/or occlusal radiographic studies.
In addition to the wide range of possible radiographic presentations, the histologic findings of osteosarcoma also display a considerable amount of variability. The vast majority of conventional osteosarcomas are classified as osteoblastic, chondroblastic, or fibroblastic. The division of the specific histologic type is based on the predominant histologic pattern of the tumor. To make a diagnosis of osteosarcoma, the direct production of osteoid by malignant mesenchymal cells must be present. In addition to osteoid, the tumor cells may produce cartilaginous matrix (chondroblastic osteosarcoma) or a high-grade spindle cell stroma (fibroblastic osteosarcoma). If the majority of the tumor is composed of an osteoid product, then it is classified as osteoblastic osteosarcoma. Chondroblastic osteosarcoma is the most common histologic type seen in the jaws. The grading of osteosarcoma is based on the amount of cellular atypia; they typically graded on a scale of 1 to 4, with 4 being the highest grade (3-5).
Osteosarcoma is a persistent malignancy. Its most important prognostic indicator is the ability to obtain initial complete surgical removal. Untreated, conventional osteosarcoma is universally fatal. Osteosarcoma has an aggressive local growth potential and a propensity to spread systemically via hematogenous routes. The lung is the most frequent site of metastasis. Metastases from mandibular lesions are more frequent than those from maxillary lesions. Today, therapy is typically multi-disciplinary, focusing on both local and systemic manifestations of osteosarcoma, thus incorporating surgery and chemotherapy (3). The use of this combined approach has resulted in a survival rate of 60-80%. In a 1997 study performed at the University of Washington, patients diagnosed with head and neck osteosarcoma had an overall 5-year survival rate of 72% (4). The suppuration with cutaneous fistula can be seen with advanced osteosarcoma. The histology, however, is not supportive of such diagnosis.
Sorry! you are incorrect
It is very rare for chondrosarcoma to cause such suppuration and cutaneous fistula. The latter is more commonly seen with periapical abscesses breaking through the bone into the soft tissue in the form of osteomyelitis. Chondrosarcoma is a malignant mesenchymal neoplasm of cartilage origin. It is uncommon, especially in the jaws. However, it is still far more common than the benign counterpart (chondroma) in the jaws. It occurs more often in the maxilla, particularly in the incisor area. It can occur at any age, but it is most common in males in the sixth decade of life (1-2). Unlike osteosarcoma, it has a low tendency for metastasis. In general, the prognosis is better than that of osteosarcoma, but it can vary depending on the stage of the disease. It commonly presents as an asymptomatic swelling with buccal and lingual expansion. Patients may experience unexplained parasthesia, headache, loosening and loss of teeth. Radiographically, it presents as an ill-defined, mottled radiolucency with snowflake or punctate calcifications (1-2). Sometimes, the teeth will show a widened periodontal ligament space. Histologically, it is characterized by immature and pleomorphic cartilage, but at times the cartilage is benign-looking. It is rare for the cartilage to calcify or differentiate and form bone (1-2). Therapy for chondrosarcoma ranges from wide local excision to radical resection with or without chemotherapy, depending on the stage of disease. The prognosis ranges from good to poor. The suppuration with cutaneous fistula is unusual for chondrosarcomas but it can happen. The histology in this case is not supportive of chondrosarcoma.
Sorry! you are incorrect
Cancer metastasis to the posterior mandible is rare and constitutes less than 1% of all oral malignant neoplasms. Theoretically, any malignant neoplasm can metastasize to the oral cavity, but in actuality few do and out of the ones that do, the majority are carcinomas rather than sarcomas. The most common malignant neoplasms that metastasize to the mouth are from the breast, lung, kidney and prostate (6-7). Breast cancer is the most common neoplasm to metastasize to the oral cavity regardless of gender. Lung and prostate cancers are the most common neoplasms to metastasize to the oral cavity in men. In most cases, the oral presentation is a secondary diagnosis where the primary diagnosis of the distant organ has been already made and the patient has had or is undergoing treatment for it. Although rare, it is known that on occasion, the oral lesion is the first manifestation of the disease. By far the most common location is the posterior mandible, where 80% of cases occur, followed by the gingiva. It is mostly described in adults over the age of 30 and rarely in children. Pain and swelling are the most common clinical symptoms. Tooth loosening, displacement and sharp resorption have also been described. The radiographic appearance of irregular bone destruction, pathologic fracture and combined radiopaque and radiolucent lesions is common for metastatic neoplasms to the mandible. Therefore, fistulas with suppuration in an area of malignant neoplasms (primary or metastatic) are not unusual. The majority of neoplasms cause bony destruction with ill-defined borders, the moth-eaten appearance of some bony destruction indicating aggressive behavior. Patients are typically treated with chemotherapy and the five-year survival rate is very low. The histology was not supportive of a metastatic malignant neoplasm.
Congratulations! You are correct
Fosamax was first introduced into the market for the treatment of osteoporosis and osteopenia in 1995. It has since been dispensed in 147 millions prescriptions. It is nitrogen containing bisphosphonates (BSPs); the latter is a synthetic analog of inorganic pyrophosphate known for its anti-osteoclastic activity. Only 1% of orally ingested BSPs are deposited in the bone compared to 50% of the IV BSPs. This chemical has a P-C-P bond rendering it indigestible and for that reason, once taken, it stays in the bone for many years if not for the life of the patient. It is released by the bone during resorption only to be recycled back into the bone but at a lower concentration. For example if a patient was on Fosamax for 10 years, he/she would recycle Fosamax at 25% of the original dose for many years (half life for Fosamax is 12 years) without a single new pill taken by the patient. The bone metabolism of patients taking Fosamax may remain suppressed for many years depending on the dose and duration of intake. One study shows that when bone was examined after five years usage, then discontinued for five years, markers of resorption and formation were still suppressed. The usual dose of 10 mg per day suppresses bone forming surface by 60-90%. However, one report indicates that the profound suppression is partially reversible in that treated bones are still able to show a blunted anabolic response to intermittent PTH.
Long-term use is controversial; there are investigators who warn against Fosamax use beyond five years and those who advocate indefinite use or at least 10 years use. For example Ott describes results of a study involving patients who used Fosamax for 4 to 7 years. In evaluating the rate of skeletal fractures, particularly vertebral fractures, Ott states that there is an increase in bone strength and a decrease in fracture rate in patients taking the medication for up to four years. However, after the four years, she states that there is a profound suppression of bone formation leading to a negative effect on the bone. A major study on Fosamax involving 247 postmenopausal osteoporosis female patients lead by Bone et al and supported by Merck Research Laboratories showed that 10 mg/day for 10 yrs resulted in increased bone density of 13.7% at the lumbar spine, 10.3% at the trochanter, 5.4% at the femoral neck and 6.7% at the proximal femur. The authors stated that Fosamax is effective and has no loss of benefits regarding fracture and stature. This study, however, makes no mention of oral osteonecrosis, a complication observed in the dental literature.
The mechanism of action of BSPs-induced bone necrosis is multifactorial and involves inhibition of the osteoclastic activity. This inhibition would affect the activity of the osteoblasts and change the environment for the endothelial cells, thus affecting angiogenesis and adversely interacting with the periodontal ligament cells. During treatment for osteoporosis with Fosamax, markers of bone remodeling show a decline of both urinary N-telopeptides (NTX) and blood C-telopeptides (CTX) of type I collagen and bone-specific Alkaline phosphotase in serum from elevated values to pre-menopausal levels. Marx et al has suggested that a certain concentration of CTX can be used as a factor in determining bone in the jaw allowing the surgeon to decide whether or not to perform implants. Without a proper clinical study the author is weary of such suggestion especially when CTX is not specific to the jaw bones.
It has been less than three years since bisphosphonates were first reported to be associated with jaw osteonecrosis (BON); and to date hundreds of cases are reported worldwide. It is, however, important to emphasize that by far the IV BSPs are most responsible for the jaw osteonecrosis and that incidence of BON is much lower with oral BSPs including with Fosamax. It is widely known that osteonecrosis is dependent on the type (nitrogen containing more potent), route of administration (IV more potent), dosage (the higher the dose the more likely to develop osteonecrosis) and on the duration of use (the longer the use, the more likely to develop osteonecrosis).
Jaw osteonecrosis is also known to occur in environments other than the use of BSPs. It has been described in association with radiation therapy to the head and neck area, with chemotherapy, especially with excessive steroid use, diabetes, herpes zoster, transplant, trauma, and in patients with bone cancer, including primary and metastatic cancers without the use of BSPs. It is worth noting, however, that the number of jaw osteonecrosis cases increased since the BSPs active clinical use, especially in multiple myeloma where up to 10% of the patients develop it. According to the company producing Fosamax (Merck), less than 1 in 100,000 patients develop BON. The literature contradicts the company numbers; one study reported that 10% of their BON patients were on oral BSPs (predominantly Fosamax). It is obvious that the number of patients with osteonecrosis who are taking Fosamax is much less than that of the IV BSPs; but it is neither as low as the company claims, nor as high as 10%. Long-term studies are needed to arrive at a more scientific conclusion as to the real incidence.
Although BON is increasingly being reported, soft tissue and mucosal lesions such as non-healing oral ulcers, spontaneous periodontitis, dental abscess, poor healing, and unexplained infections have been also described several years before the jaw osteonecrosis was identified. Other symptoms range from jaw numbness, pain, and feeling heavy, to frankly necrotic and oozing pus, as is the case in this patient. The most clinically described symptom is non-healing extraction site in about 60-80% of the time especially true in the IV BSPs.
Risk factors thus include extraction, periodontal surgery or any other surgical manipulation of the bone. Mucosal breakdown and ulceration of the bone-bound mucosa exposing the bone to the outside environment can be the beginning of the bone necrosis. Other risk factors include: the age of the patient, whether or not he/she is on chemotherapy or steroids, has primary or metastatic bone cancer, is wearing ill-fitting dentures, has a periapical lesion, or has badly carious or partially erupted teeth. Other risk factors would include large tori and exostoses as well as implants.
Osteonecrosis is rarely described in bones other than the jaws. The etiology centers on poor local vascularity and impaired wound healing, especially in the posterior mandible and in the lingual posterior mandible where the bone is thick, more lamellar and less vascular. Olson et al suggest that it is most common in the mandible because of the nature of the blood vessels and the anatomy of the bone. They state that blood flow in the end arteries of the posterior mandible is weak and the bone is thick. They further suggest that trauma, be it from mastication, surgery, extraction or vasoconstrictor containing anesthesia are common in the jaws. When trauma is combined with poor healing and poor vascularity bone necrosis occurs. Also, an added problem is the constant exposure to insults and infection from the oral cavity that render it more susceptible to infection and constant remodeling. The mandible and maxilla are the only bones connected to an exterior housing teeth and periodontium with their complication and vulnerability to trauma and infection.
Treatment of BON has proven to be difficult, especially when it occurs while the patients are on BSPs. This is especially true with the IV BSPs. The treatment of choice is non-aggressive removal of the sequestered bone, infection control with antibiotics and twice-a-day chlorhexidine rinses and pain control medications. Resection is rarely used as a mode of treatment, which was the case in this patient. The overall philosophy of treatment is to treat patients as if undergoing radiation and to perform a full-mouth treatment prior to placing the patients on Fosamax or any other BSPs. Surgery involving jaw bone of any should be avoided; extraction should be replaced with endodontic treatment and mild to moderately mobile teeth splinted rather than extracted. If extraction is inevitable, the area should be handled gently with minimum damage to the soft and hard tissue; the area should be sutured and the patient should be placed on antibiotics (for two weeks to start and more necessary) and chlorhexidine rinses (for at least two months). An Expert Panel representing the American Dental Association produced an excellent protocol for prevention and treatment of BON and should be consulted if treating patients on BSPs (please see attached the complete report).
Treatment
Under local anaesthesia the area was originally treated with gentle irrigation with chlorhexidine and gentle curettage of the sequestered bone with suture of the overlying soft tissue. The patient was also treated with several courses of antibiotics (amoxicillin-clavulanic acid 875 mg, three times per day). The area did not respond to the treatment and the sequestration recurred with pain and a small oral fistula. The condition slowly progressed to increased pain and additional problems with normal feeding. Biopsy specimen excluded malignant neoplasm and confirmed the diagnosis of osteomyelitis. Local curettage, chlorhexidine rinses and antibiotic were not successful in eliminating the symptoms. Because of the persistence of the disease, the area was resected under general anaesthesia (Fig 3). This is a very unusual treatment modality for BON, but it has been used in osteoradionecrosis. The reason is that the jaw bones in BSPs users are all affected, while in osteoradionecrosis the affected areas are localized to the irradiated site. The jaw defect was replaced with a titanium plate. A 12 -month follow-up reveals the area to be healing well with no symptoms or recurrences. The patient can also eat without problems.
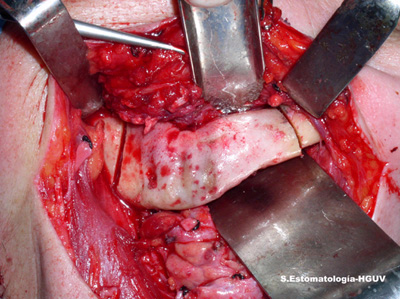
Figure 3. This is a clinical photograph demonstrating the surgical resection of the necrotic bone. This mode of treatment is unusual for bisphosphonates induced osteonecrosis.
References
- Anil S, Beena VT et al. Chondrosarcoma of the maxilla. Case report. Aust Dent J. 1998;43:172-174.
- Hayt MW, Becker L et al. Chondrosarcoma of the maxilla: panoramic radiographic and computed tomographic with multiplanar reconstruction findings. Dentomaxillofac Radiol. 1998;27:113-116.
- Bennett JH, Thomas G et al. Osteosarcoma of the jaws: a 30-year retrospective review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:323-332.
- Oda D, Bavisotto LM et al. Head and neck osteosarcoma at the University of Washington. Head Neck 1997; 9:513-523.
- Slootweg PJ, Muller H. Osteosarcoma of the jaw bones. Analysis of 18 cases. J Maxillofac Surg 1985;13:158-166. A.
- Hirshberg A and Buchner A. Metastatic tumours to the oral region. An overview. Oral Oncol. 1995; 31: 355–360.
- Ivan der Waal, RIF, Buter, J. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg. 2003; 41: 3-6.
- Uhler IV, Fahs GR, Dolan LA. Metastasis of cervical carcinoma to the mandible: report of a case. J Am Dent. Assoc. 1972; 85: 363-364.
- Bagan JV, Jiminez Y, Murillo J, et al. Jaw osteonecrosis associated with bisphosphonates: multiple exposure area and its relation to teeth extractions. Study of 20 cases. Oral Oncol.
- Bagan JV, Murillo J, Jiminez Y, et al. Avascular jaw osteonecrosis in association with cancer chemotherapy: series of 10 cases. Oral Pathol Med 34;120-3, 2005.
- Bone HG, Hosking D, Devogelaer J-P, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N E J Med 350;1189-99, 2004.
- Ficarra G, Bentinati F et al. Osteonecrosis of the jaws in patients with a history of bisphosphonates treatment. J. Clinical Periodontology. 32: 1123-1128, 2005.
- Hellstein JW, Marek CL. Bisphosphonare osteochemonecrosis (bis-phossy jaw): is this phossy jaw of the 21st century? J Oral Maxillofac Surg. 63;682-9, 2005.
- Marx RE, letter.Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws; a growing epidemic. J Oral Maxillo Surg 61;1115-17, 2003.
- Marx RE, Sawatari Y, Fortin M, Broumand V. Biphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention and Treatment. J Oral Maxillofac Surg 63:1567-75, 2005.
- Migliorati CA, Schubert MM, Peterson DE,Seneda M. Biphosphonate-associated osteonecrosis of mandibular and maxillary bone. Cancer 104;83-93, 2005.
- Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endo Metab 90;1294-1301, 2005.
- Olson KB, Hellie CM, Pienta KJ. Osteonecrosis of jaw in patient with hormone-refractory prostate cancer treated with zoledronic acid. Urology 66;658-60, 2005.
- Ott SM, Editorial. Long-term safety of bisphosphonates. J Clin Endo Metab 90;1897-99, 2005.
- Woo SB, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 16: 144: 735-61, 2006.
- Wood J, Bonjean K, Ruetz S, et al. novel antiangiogenic effects of the bisphosphonates compound zoledronic acid. J Pharmacol Exp Ther 302;1055-61, 2002.