April 2009: Large gingival swelling, left posterior mandible
Can you make the correct diagnosis?
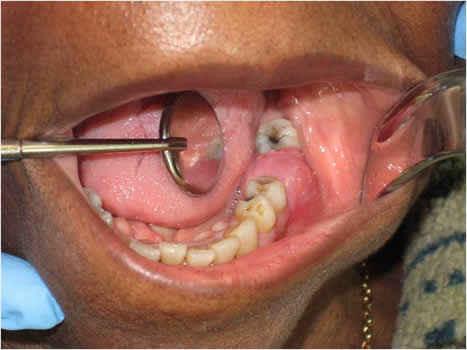
This is an 83-year-old black female who presented to the emergency clinic at Harborview Medical Center with gingival swelling and pain in the left posterior mandibular alveolar ridge between teeth #s 18 and 20; tooth # 19 was missing (Figure 1). The patient stated that the area was painful, especially when biting down. The area had been sensitive for several weeks and had been swollen for a few months. The panoramic radiograph showed ill-defined radiolucency in the area of missing tooth #19 (Figure 2). The swelling measured 2 x 2 centimeters with 1.5-cm height. There was no history of tobacco or alcohol use.
Sorry! you are incorrect
The most common cause of gingival swelling is a reactive process and for that reason, it would be necessary to include reactive gingival swellings such as pyogenic granuloma (PG), peripheral ossifying fibroma (POF) and peripheral giant cell granuloma (PGCG) on the differential diagnosis. They can be fast growing and can be painful, especially during eating and if the surface is ulcerated. The color is not supportive of PG or PGCG. The histology was not supportive of any of the reactive gingival swellings.
Pyogenic granuloma constitutes 85% of all reactive gingival swellings, representing a profuse mass of vascular granulation tissue (1-3). It can be induced by local irritants such as excessive plaque, sharp fillings and dental calculus; it sometimes forms in an extraction socket in response to an irritant left in the socket. It can occur anywhere in the oral cavity and skin, especially the tongue, lips, fingers and nail beds (1-3). In the mouth, it occurs most commonly in the gingiva, especially the maxillary buccal and interproximal gingiva (1-2). Occasionally, it may surround the tooth. It is usually highly vascular, fast-growing, exophytic, lobular, sessile, and ulcerated or covered by pseudomembrane. The color changes from red to pink when it starts to heal. It occurs at any age and sex with a slight predilection for young females; it affects 1% of pregnant females. Pyogenic granuloma is usually painless except during eating, when bleeding and pain is described (1). Peripheral giant cell granuloma constitutes less than 5% of all reactive gingival swellings, and consists of a hyperplastic mass of vascular granulation tissue with many osteoclast-like multinucleated giant cells. It presents as a lobular, purplish-blue exophytic nodule exclusively on the gingiva, both edentulous and dentate, and usually anterior to the molars (4-5). It originates from either the periodontal ligament or the periosteum. It occurs across a wide age range, especially in children, young adults, and females (2:1 female to male ratio) (4-5). It presents as either sessile or pedunculated and smooth surfaced or lobular; though usually painless, it can occasionally be ulcerated, painful and accompanied by bleeding (4-5). Like pyogenic granuloma, it is usually present either on the buccal or lingual gingiva or between teeth, but it can occasionally surround the teeth (4-5) and act aggressively by displacing teeth much like a sarcoma (4). It can also resorb the underlying bone in a smooth and concave “saucer-like” manner. Complete excision including curettage of underlying bone is the preferred treatment. It has a good prognosis with a recurrence rate of approximately 10% (5). Peripheral ossifying fibroma constitutes 10% of all reactive gingival swellings. It consists of a moderately cellular fibrous connective tissue mass with bony trabeculae and/or cementum-like hard tissue. It has occasionally been reported to occur on edentulous alveolar mucosa. It originates from the periodontal ligament or the periosteum. This lesion is most common in young patients between 1 and 19 years of age and has a predilection for females over males by a 3:2 ratio (6-8). It occurs exclusively on the gingiva, especially the anterior gingiva, with slight predilection to the maxilla and rare presentation in primary teeth (8). It is usually sessile and exophytic and often ulcerated; it presents as well-demarcated sessile nodules, which are firm or hard depending on the amount of ossification and calcifications (7-8). Peripheral ossifying fibroma is usually pink but can be focally red if ulcerated. Deep surgical excision to include the periodontal ligament is the preferred treatment, though laser removal has been used effectively. Deep surgery may lead to a gingival defect, which would require gingival grafting, especially if it is located on the anterior buccal gingival (8). There is a 16-20% recurrence rate (8).
Sorry! you are incorrect
The differential diagnosis of fast growing soft tissue destroying the alveolar bone in an ill-defined manner should include aggressive conditions and neoplasms including aggressive fibromatosis and some sarcomas. The histology, however, was not supportive of aggressive fibromatosis or a sarcoma.
Aggressive fibromatosis represents a group of fibrous neoplasms with various clinical behaviors and histologic presentations. However, it is considered to be a benign but locally infiltrative and aggressive neoplasm; it has a potential for multiple local recurrences, but no potential for metastasis. Aggressive fibromatosis can be sporadic or familial, such as those associated with Gardner’s syndrome, which tends to occur more in the abdominal area, and rarely in the head and neck area (9-11). Head-and-neck aggressive fibromatosis usually occurs in children under 20 years of age (9). It is more prevalent in adult females by a ratio of 2:1; in children, it is more common in males by a 2:1 ratio (10-11). It typically presents as a firm, painless, rapidly growing asymptomatic mass. Occasionally, it is slow-growing, but it is destructive in either case. It can destroy bone, infiltrate adjacent structures, displace teeth, and induce periosteal reaction, rendering it clinically and radiographically difficult to differentiate from a sarcoma. It presents in multiple locations within the oral cavity, including the gingiva, lips and other oral soft tissue areas. There is a legitimate body of evidence to suggest that estrogen promotes the growth of fibromatosis, particularly in pregnant females (9). Association with the FAP (Gardner’s Syndrome) gene is also well documented (10). The clinical behavior of aggressive fibromatosis is unpredictable and ranges from frequently recurring to spontaneously regressing and for that reason, a variety of treatment modalities have been employed. Surgery, which is the treatment of choice, ranges from complete removal with clean margins to debulking of the lesion to avoid loss of vital structures. The latter method is often necessary for oral cavity lesions. Several non-surgical alternative treatments have also emerged, such as radiotherapy, chemotherapy, hormonal and biological manipulation, and non-steroidal anti-inflammatory agents. Surgery combined with radiotherapy or chemotherapy has been used in recurring and persistent lesions. Radiotherapy is a secondary treatment method to surgery, and is sometimes used alone (11). Some cases have been successfully treated with estrogen antagonists and non-steroidal anti-inflammatory drugs (9). Prognosis and recurrences are difficult to predict. The recurrence rate ranges from 20% to 70%, mostly recurring within the first five years (10). Some studies suggest that positive margins may be the only predictor for recurrence, if any (11). Other adverse prognostic factors may include age of less than 18 years, recurrent disease, and surgical treatment alone (10-11).
Sorry! you are incorrect
Given the age and the bony changes of ill-defined radiolucency, one should consider a malignant neoplasm on the differential diagnosis. Because squamous cell carcinoma is the most common malignant neoplasm of the oral cavity, it should be included on the differential diagnosis. However, the clinical presentation of intact mucosa with no ulceration or red or white lesion should render such a diagnosis unlikely. The histology was not supportive of gingival squamous cell carcinoma.
Squamous cell carcinoma (SCC) of the gingiva is uncommon, especially in a non-smoker and non-alcohol user. Gingiva constitutes an area of 12% of oral SCCs. Oral SCC ranks as the fifth most common malignant neoplasm worldwide and accounts for an estimated 90% of oral malignancies (12-15). Oral SCC occurs predominantly in males over the age of 40 years, with an observed male-to-female ratio of 2:1 generally and 1.4:1 in the USA (12-14). Excluding the outer lip, the most common sites (in decreasing order) are ventral and lateral surfaces of tongue (25-50%), floor of mouth (15%), gingiva (12%) and palate (9%). The buccal mucosa and retromolar pad areas (3%) have a relatively low incidence of occurrence (13-15) unless the patient is a chronic smokeless tobacco user. Oral SCC varies in presentation from deceptively innocent-looking to obviously malignant. It may present as a non-healing ulcer, or as red, white or mixed red-and-white lesions. Characteristic signs of oral SCC are non-healing ulcer, ulcer with rolled borders, fungation, fixation and induration. Rarely, oral SCC may present as unexplained asymptomatic lateral neck lymphadenopathy (12-14). Oral SCC is most commonly associated with chemically induced mutagenesis, specifically tobacco and alcohol use (11).
Tobacco use is described in over 75% of oral SCC patients (14). Tobacco and alcohol have been shown to act synergistically in the development of oral SCC (12). Human papilloma virus (HPV) has also been found to have a high prevalence in oral cancer, especially in younger patients with no history of tobacco use (12). Other factors include poor oral hygiene, syphilis, chronic candidiasis, iron and dietary deficiencies, herpes simplex and various other immunologic factors (12), and lichen planus—especially the persistent erosive lichen planus (13).
Determination of the prognosis of OSCC is based on its clinical stage using the TNM classification. The prognosis improves when the lesion is detected early. Oral SCC patients die mainly of infection or of hemorrhage if the tumor erodes through one of the main blood vessels.
Congratulations! You are correct
Multiple myeloma is a clonal neoplastic proliferation of plasma cells (myeloma cells) which are terminally differentiated B-lymphocytes. It is most common in individuals 60-75 years of age, more in males, and rare in persons under 40 years of age (16-19). It is twice as common in blacks as it occurs most commonly in bone and in a multicentric manner (multiple locations at the same time), thus the name multiple myeloma (16-17) This malignant neoplasm presents more commonly in bone but is also described in soft tissue without bony involvement (extramedullary). It can also present as an isolated lesion which is called plasmacytoma. About 40-50% of bone-isolated plasmacytomas will progress to multiple myeloma while only 10-12% of soft tissue plasmacytomas will progress to multiple myeloma (16-19). Normal plasma cells are immunoglobulin producing cells including IgA, IgD, IgG, IgM, and IgE. Neoplastic plasma cells tend to produce any of these antibodies but predominantly IgG and IgA. The most common clinical presentation is severe bone pain, especially that of the lumbar spine which is usually aggravated with movement (16-18). Bone lesions occur in 70% of patients and usually lead to hypercalcemia, bone fracture and vertebral collapse. Hypercalcemia may lead to soft tissue calcifications. The most common bones affected by multiple myeloma are the vertebrae, skull, mandible, ribs, pelvis, clavicles and scapula. Jaws are affected in about 30% of cases (17-19). Extramedullary tissue such as the liver, spleen and lymph nodes can also be affected. Patients with multiple myeloma are susceptible to anemia and therefore often experience fatigue and weakness. They can also develop renal failure due to monoclonal light chain (Bence Jones protein). About 15% of patients develop amyloid deposition in a variety of soft tissues, including the G-I tract and the oral cavity, specifically the tongue. Amyloid in the tongue can present as small yellow nodules or plaques; they can be deep seated within the tongue, causing slurring. They can also occur around the eyes, again as small yellow plaques or nodules (16). Radiographically, multiple myeloma has a distinct presentation of “punched out” radiolucencies but the radiolucency can also be ill defined. This is particularly true of the skull lesion. Mandible and maxilla can be affected, but the radiographic presentation is not as distinct as of the skull lesions (16-19). It is important to note that histology and immunohistochemistry alone cannot differentiate between isolated case of plasmacytoma and the full diseases of multiple myeloma. Pathologists usually render the histologic diagnosis of plasmacytoma while the oncologists render the clinical diagnosis of multiple myeloma.
Histologically, the biopsy of a patient with isolated plasmacytoma or a patient with the full disease of multiple myeloma demonstrates sheets of monotonous mononuclear cells with plasmacytic differentiation. The atypical plasma cells usually show nuclear and cellular atypia. Mitosis is usually present (17-18). The neoplastic cells are monoclonal; this can be supported by an immunohistochemistry stain with kappa or lambda light chains. They are also specific with CD138, a marker for plasmacytoid cell. The final diagnosis of multiple myeloma is determined through a combination of histology, serum and urine protein electrophoresis, blood test for myeloma protein (monoclonal gammopathy), and radiographic changes. Treatment includes chemotherapy, bisphosphonates to control hypercalcemia, bone marrow transplant and radiation therapy. The overall prognosis depends on age; younger patients have a better prognosis than older patients.
References
- Jafarzadeh H, Sanatkhani M, Mohtasham N. Oral pyogenic granuloma: a review. J Oral Sci 2006; 48:167-75.
- Fantasia JE, Damm DD. Red nodular lesion of tongue. Pyogenic granuloma.
Gen Dent. 2003 Mar-Apr;51(2):190, 194. - Ichimiya M, Yoshikawa Y, Hamamoto Y, Muto M. Successful treatment of pyogenic granuloma with injection of absolute ethanol. J Dermatol. 2004 Apr;31(4):342-4.
- Flaitz CM, Peripheral giant cell granuloma: a potentially aggressive lesion in children.
Pediatr Dent. 2000 May-Jun;22(3):232-3. - Chaparro-Avendano AV, Berini-Aytes L, Gay-Escoda C. Peripheral giant cell granuloma. A report of five cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2005 Jan-Feb;10(1):53-7; 48-52.
- Hanemann JA, Pereira AA, Ribeiro Junior NV, Oliveira DT. Peripheral ossifying fibroma in a child: report of case. J Clin Pediatr Dent. 2003 Spring;27(3):283-5.
- Walters JD, Will JK, Hatfield RD, Cacchillo DA, Raabe DA. Excision and repair of the peripheral ossifying fibroma: a report of 3 cases. J Periodontol. 2001 Jul;72(7):939-44.
- Cuisia ZE, Brannon RB. Peripheral ossifying fibroma–a clinical evaluation of 134 pediatric cases. Pediatr Dent. 2001 May-Jun;23(3):245-8.
- Donahue WB, Malexos D, Pham H. Aggressive fibromatosis of the maxilla. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1990; 69:420-426.
- Jones IT, Jagelman DJ, et al. Desmoid tumors in familial polyposis coli. Ann Surg. 1986; 170:109-121.
- Fletcher CDM. Diagnostic Histopathology of Tumors. Third edition, Volume 1. 2007; pages 92-93.
- Bundgaard T, S Bentzen, et al. Histopathologic, stereologic, Epidemiologic, and clinical parameters in the prognostic evaluation of squamous cell carcinoma of the oral cavity. Head & Neck. 18:142-152 (1996).
- Barasch A, DE Morse, et al. Smoking, gender, and age as risk factors for site-specific intraoral squamous cell carcinoma. Cancer 73:509-513 (1994).
- Syrjanen SM, KJ Syrjanen et al. Human papillomavirus (HPV) DNA sequences in oral precancerous lesions and squamous cell carcinoma demonstrated by in situ hybridization. J Oral Pathol. 17:273 (1988).
- Holmstrup P, JJ Thorn, et al. Malignant development of lichen planus-affected oral mucosa. J Oral Pathol. 17:219-25 (1988).
- Akhtar K, Laghari NA, Haq AU, Anees M, Rehman SU, Alam MI. Multiple myeloma in younger age.. J Coll Physicians Surg Pak. 2009 Jan;19(1):62-3
- Poggio CE. Plasmacytoma of the mandible associated with a dental implant failure: a clinical report. Clin Oral Implants Res. 2007 Aug;18(4):540-3. Epub 2007 Apr 30.
- Canger EM, Celenk P, Alkan A, Günhan O. Mandibular involvement of solitary plasmocytoma: a case report. Med Oral Patol Oral Cir Bucal. 2007 Jan 1;12(1):E7-9.
- Souza LN, Farias LC, Santos LA, Mesquita RA, Martelli H Jr, De-Paula AM. Asymptomatic expansile lesion of the posterior mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Jan;103(1):4-7. Epub 2006 Oct 16.