Diffuse white wrinkled lesion buccal mucosa and vestibule
Can you make the correct diagnosis?
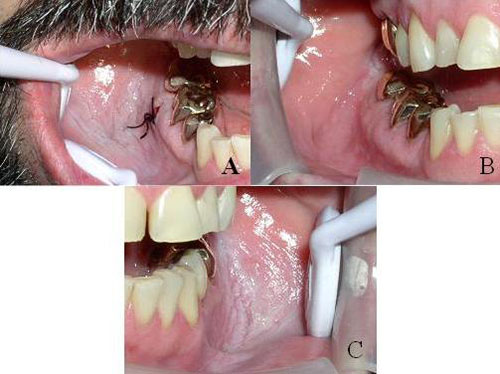
This is a 46-year-old male who presented with a lesion on the right buccal mucosa, from the linea alba through the buccal vestibule to the area of teeth #s 30 and 31. The buccal mucosa appeared white and wrinkled from the mesial of tooth #29 to the distal of tooth #31 (Figure 1A). The lesion was first noticed by the patient’s general dentist in 1999, at which time it was white, wrinkled, soft, non-fixed and non-tender. At both presentations, there were no palpable masses, and head and neck examination was otherwise within normal limits.
Congratulations! You are correct
Tobacco products are available in two forms: smoked or smokeless. The latter can come in the form of plug, chewing tobacco, or dry or moist snuff. Dry snuff can be inhaled or placed between the lip or cheek and the gingiva. Smoked tobacco is used in cigarettes, cigars or pipes (5).
Smokeless tobacco keratosis is the term for white plaques that form on the oral mucosa, usually vestibule, and the gingiva in the areas which habitually come into direct contact with tobacco in a smokeless tobacco user (1-5). These lesions are characterized by thickened, white mucosa that is typically wrinkled. This type of lesion takes between 1 and 5 years to develop depending on the total hours of daily use and the amount of use (1-5). The clinical presentations of these lesions go through stages; it usually presents as an ill-defined area of white and wrinkled thickening most commonly on the mandibular labial and buccal mucosal folds, but the early presentation can be less prominent and pink in color and nonetheless corrugated and rough in palpation. More advanced lesions may have an edematous look and are again wrinkled. These lesions may also have red patches, which may indicate a change to a more serious disease, though this is rare. In addition to the mucosal and gingival changes, the teeth may also demonstrate changes including gingival recession and caries (1-5). The latter depends on the sugar content of the smokeless tobacco.
The habit of smokeless tobacco use is more common in white blue-collar males with an age range of 9-30. It occurs in the area where tobacco is held. The histology ranges from benign to low-grade dysplasia. High-grade epithelial dysplasia and squamous cell carcinoma are described, but rarely (4-5). Given this information, it has been suggested that smokeless tobacco is a safer alternative to smoked tobacco (3-5); as a result, some even believe that it can be used as an effective agent in smoking cessation. This notion is supported by several studies. For example, studies from Sweden in Europe and West Virginia in the United States, where smokeless tobacco use is common (in Sweden 20% of the young population use it), show that these areas have comparable rates of oral cancer when compared to otherwise similar areas in which smokeless tobacco use is less common. This information indicates that smokeless tobacco use is not implicated in oral cancer occurrence. However, there is still an important body of evidence in the literature to suggest that smokeless tobacco is indeed a risk factor in oral cancer (4-5), even without the carcinogenic additives that are present in the Asian and African forms. In an alternative interpretation of the data from Sweden, Warnakulasuriya (5) contends that the oral cancer occurrence rate among men in Sweden is higher than that in England, which notably has a lower rate of smokeless tobacco use and a higher rate of smoked tobacco use among men. Of additional concern is smokeless tobacco’s documented potential as a “gateway” to indubitably harmful smoked tobacco use. The Surgeon General of the United States declared in 1986 that chewing tobacco use increases the risk of oral cancer, and the current US Surgeon General maintained in a congressional testimony in 2003 (4) that smokeless tobacco is not a safe alternative to cigarettes. This author believes that smokeless tobacco is carcinogenic and does not advocate its use, be it recreational or otherwise.
Treatment of smokeless tobacco keratosis includes prohibition of tobacco use of any type. The lesion may regress with discontinuation of tobacco use within three months and may disappear within a year. Excision with clean margins is indicated if there is evidence of dysplasia; complete excision is necessary if it appears in high risk locations such as the floor of mouth or the ventral surface of tongue. Long-term follow up is mandatory. The prognosis depends on the histology and ranges from good to poor.
Treatment
The two incisional biopsies were performed to establish a baseline diagnosis. Both biopsies showed typical changes induced by smokeless tobacco use. Both times, the patient was advised to consider tobacco cessation. He tried but failed the first time. After the first biopsy, the area reverted back to normal morphology after two weeks of tobacco cessation (Figure 2B ); after the second biopsy, the patient reduced tobacco use by 25%, which lead to significant clearing of the lesion except in the retromolar pad area (Figure 1B).
References
- Wright JM. Oral and maxillofacial pathology case of the month. Snuff dippers keratosis or snuff pouch. Tex Dent J. 2003 Dec;120(12):1181, 1186-7.
- Taybos G. Oral changes associated with tobacco use. Am J Med Sci. 2003 Oct;326(4):179-82.
- Luomanen M, Tiitta O, Heikinheimo K, Heinaro I, Happonen RP. Effect of snuff on cytokeratin expression in oral vestibular sulcus epithelium. J Oral Pathol Med. 1997 Mar;26(3):110-6.
- Carmona, R. Can Tobacco Cure Smoking? A Review of Tobacco Harm Reduction. Surgeon General. 2003, June 2.
- Warnakulasuriya S. Smokeless tobacco and oral cancer. Oral Diseases. January 2004; vol 10: 1-6.
- Canaan TJ, Meehan SC. Variations of structure and appearance of the oral mucosa. Dent Clin North Am. 2005 Jan;49(1):1-14, vii.
- Martin JL. Leukoedema: an epidemiological study in white and African Americans.
J Tenn Dent Assoc. 1997 Jan;77(1):18-21. - Terrinoni A, Candi C, Oddi S, Gobello T, Camaione D, Mazzanti C et al. A glutamine insertion in the 1A alpha helical domain of the keratin 4 gene in familial case of white sponge nevus. J I Dermat 2000;114:388-391.
- Cannon AB. White sponge nevus of the mucosa. Arch Dermat and Syph 1935;31:365-370.
- Buckholz F, Schubert C, Lehmann-Willenbrock W. White sponge nevus of the vulva. Int J Gyn Obstet 1985; 23:505-507.
- Silverman S, Gorsky M et al. A retrospective study of findings and management in 214 patients with oral lichen planus. Oral Surg Oral Med Oral Pathol 1991; 72:665–670.
- Silverman S. Oral lichen planus: a potentially premalignant lesion. J Oral Maxillofac Surg 2000; 58:1286–1288.
- Duffey DC, Eversole LR et al. Oral lichen planus and its association with squamous cell carcinoma: an update on pathogenesis and treatment implications. Laryngoscope. 1996; 106:357-362.
- Pindborg JJ, Reichart PA et al. WHO International histological Classification of Tumours. Histological typing of cancer and precancer of the oral mucosa. , Springer, Berlin (1997).
Sorry! you are incorrect
Lichen planus is a common chronic immune mediated disease affecting up to 2% of the population, and has a variety of clinical presentations, management, and predisposing factors that render it a family of diseases rather than one entity with a specific treatment modality (11, 12). Specifically, lichen planus can be immune mediated, drug induced, associated with transplant rejection (also known as graft versus host disease), or associated with galvanic effect (when two separate restorations are in opposite contact with each other). It is also seen in families, though rarely, and is known to undergo malignant transformation in about 2.5% of cases. Given the versatility of this disease, it is difficult to apply one treatment modality for all presentations (12, 13).
The most common type of LP is the chronic T-lymphocyte-mediated disease in which CD4 (early stage) and CD8 (later stages) T-lymphocytes are stimulated, releasing lymphokines such as tumor necrosis factor which lead to the destruction of the cells in the basal and parabasal cell layers (12). This type of oral LP tends to come and go, sometimes lasting a lifetime. Lichen planus of the skin usually resolves within 1-3 years while only 20% of oral LP cases resolve in that period of time. Lichen planus occurs in adults between 30 and 70 years of age with a strong female predilection (11-14). It is often associated with stressful lifestyles. Skin LP presents as purplish, pruritic papules with a white keratotic surface, commonly on the flexor surfaces of the wrists, trunk and the genitalia. Oral LP presents as keratotic and reticular (the reticular pattern is the most common type), atrophic or erosive (the erosive type is the most important clinically), or plaque-like (the hypertrophic type, which is the least common). Oral LP presents most commonly in a symmetrical manner on the bilateral buccal mucosa, followed by the tongue and gingiva. Reticular type LP is asymptomatic and presents with interlacing lines against an erythematosus bluish background; the lines are known as the striae of Wickham. Erosive type LP, on the other hand, is symptomatic—patients complain of sensitivity to hot and cold, spicy, acidic, and alcoholic food and beverages. Erosive LP presents in locations similar to those of the reticular type. At times, erosive LP can be hard to distinguish from other mucocutaneous diseases such as mucous membrane pemphigoid (MMP) and pemphigus vulgaris (PV). Hyperplastic or plaque LP is uncommon and presents as a confluent white plaque often mistaken for leukoplakia. The hyperplastic type is more common on the dorsal surface of the tongue, gingiva, and palate, making a clinical diagnosis of LP difficult at times, especially if the patient is a smoker. LP can also present on the gingiva alone as a thin and atrophic lesion known as desquamative gingivitis. The clinical differential diagnosis for desquamative gingivitis should include MMP and PV.
Lichenoid Drug Reaction, most frequently present as erosive lichen planus on the bilateral buccal mucosa. It is associated with the ingestion of a number of medications including antibiotics, antihypertensive drugs, allopurinol (gout), diuretics, antidiabetics, gold, mercury, antibiotics, antihistamines and many others. The malignant potential of oral LP is a significant clinical concern, especially in long-term erosive lichen planus patients; transformation in reticular LP has also been documented, but rarely (14). The World Health Organization (WHO) defines oral LP as a precancerous condition (14) with full understanding that the risk for transformation is around 2.5%. The locations of transformation are the buccal mucosa, tongue and gingiva—the same locations as lichen planus would usually occur.
Histologically, depending on the type, the epithelium ranges from thin and erosive to thick and keratotic (12,13). All types have a band-like infiltrate of T-lymphocytes. The basal cell layer shows evidence of degeneration; rete pegs are or are not present, the basement membrane zone is thickened, and cytoid bodies (Civatte bodies) may be present. The immunofluorescence (IMF) features include positive staining with antibody to fibrinogen present along the basement membrane. Treatment ranges from no treatment for the asymptomatic reticular type, to topical steroids, to intralesional steroid therapy (rarely used), to systemic steroid therapy (if the condition is severe such as in erosive LP). The best treatment for lichenoid drug reaction is replacement of the causing medication with a substitute. Neither the histology, nor the clinical history of this case are supportive of lichen planus.
Sorry! you are incorrect
The name white sponge nevus was coined by Cannon in 1935 (9), but the disease was described earlier by Hyde. It is a rare condition that has a dominant autosomal inheritance; typically, 50% of children of an affected parent will inherit the condition. It is a benign and usually asymptomatic (not painful) lesion that occurs at birth or during childhood or adolescence (8-10). It occurs in both sexes equally and has no race predilection (8-10). Like leukoedema, this lesion is most common in the mouth but can occur in other sites such as the nose, esophagus, vagina or rectum. This condition is believed to be due to mutations in Keratins type 4 and 13. White sponge nevus can be mistaken for leukoplakia, but the history of the presence of the condition since childhood combined with a family history of the disease and the histology are adequate for rendering a definitive diagnosis. Clinically, it presents as white, folded or corrugated with a soft and spongy consistency, or alternately as translucent, much like leukoedema. It presents as plaques on the bilateral buccal mucosa, lips, gingiva, floor of mouth and lateral tongue (8, 10). They can also occur extra-orally, especially in the vagina and rectum, but such cases are very rare in comparison to cases in the oral cavity. The corrugated or folded morphology does not flatten when the mucosa is stretched; this feature can be used to distinguish between leukoedema and white sponge nevus clinically.
Histologically, the epithelium is thickened and covered by parakeratin with keratin plugging and intracellular edema of the spinous layer. Because this condition is benign, treatment is usually not indicated and it has an excellent prognosis. This lesion is rarely symptomatic, especially in the oral cavity. However, if it is symptomatic, mouth rinses are recommended and for the genital area, if symptomatic, anti-itch medications have been recommended. The histology and clinical history of this case are not supportive of white sponge nevus.
Sorry! you are incorrect
Leukoedema is a common developmental condition that is believed to represent a variation of the normal mucosa. It is more evident in black patients, mostly because of the mucosal dark pigmentation exaggerating the milky color of this condition (6-7). It occurs most commonly in the oral cavity (6-7) but can also affect other sites, including the vagina and the larynx (6-7). It is characterized by the accumulation of fluid within the epithelial cells resulting in a soft texture and milky color. It usually presents as folded mucosa, mostly on the bilateral buccal mucosa and vestibule. Upon stretching, the wrinkles flatten and disappear, giving the clinician a tool to differentiate this entity from other white oral lesions. As mentioned above, this lesion is more common in blacks, affecting as many as 94% of black adults and 50% of children. It is also described in 40% of Caucasians. It is exaggerated in heavy smokers, but reverts back to the less prominent appearance upon cessation of tobacco use. Histologically, the epithelium shows acanthosis with cellular edema of the spinous layer. Epithelial cells look swollen with pyknotic nuclei. The surface is usually covered by parakeratin. No treatment is indicated, and it has an excellent prognosis. The clinical presentation of this lesion may suggest leukoedema; however, neither the histology nor the history of smokeless tobacco use are in support of this diagnosis.