A pair of studies led by University of Washington researchers has cast important new light on the body’s response to viral or bacterial invaders. In particular, they are learning how that immune response might be controlled to target the response more tightly and limit the collateral damage on this biological battleground.
When a microorganism or toxin enters the body, the immune system swings into action, sending white blood cells known as lymphocytes to fight the invaders. T-cells and B-cells, which are specialized forms of these lymphocytes, attack the source of infection. But in the course of this fight, the T-cells also spur inflammation, which can severely damage tissue. Some of these T-cells are also critical for tissue repair and resolving inflammation, but it has remained unclear if the balance can be tipped to favor tissue repair over inflammation.
Now, Dr. Douglas Dixon, Dr. Martin Prlic, and their research colleagues are advancing our knowledge of how to possibly tip the balance of these T-cells to limit the collateral tissue damage. Dr. Dixon is a clinical associate professor of periodontics at the School of Dentistry, and Dr. Prlic holds faculty appointments in the Molecular and Cellular Biology Program at the UW and at Seattle’s Fred Hutch Cancer Research Center.
“We’re gaining a better understanding of chronic inflammation,” Dr. Dixon said. “The possibilities include localized treatment to block inflammation or turn up the immune response, depending on the need. You can design a molecule to bind to a receptor and turn it on or off as needed.”
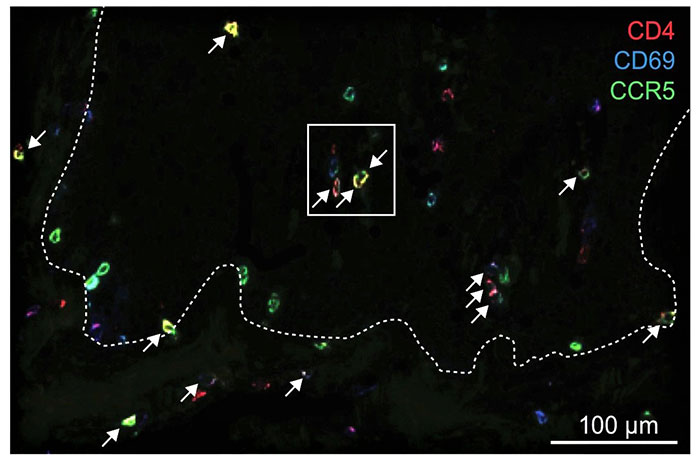
A key finding has been that while the body rushes reinforcements to the site of an attack, they may not be needed – the defenders already residing at the site may be able to handle the job. Most importantly, these defenders also have the ability to rebuild tissue. That’s what the researchers discovered in a study published in the Science Translational Medicine journal. They investigated a protein called CCR5, which acts as a GPS for cells of the immune system and guides them around tissues but can also be a “back door” for HIV to enter and spread throughout the body.
Anti-HIV drugs that work on blocking CCR5 to prevent infection also block that GPS function. However, the researchers found that the T-cells already residing in the tissue aren’t affected.
“So if you block new T-cells from entering the site of the infection, you may be able to prevent damaging inflammation while the resident T-cells handle the infection and carry out repair functions,” Dr. Dixon said.
A second study, published in the ImmunoHorizons journal of the American Association of Immunologists, focused on the MAIT cell, an unusual type of T-cell that can sense the presence of bacteria.
MAIT stands for mucosal associated invariant T-cell. When one of these cells are exposed to inflammation, they quickly generate-the protein CTLA-4, which the researchers say is basically an “off” switch for the immune response.
“Now we’re working on identifying ways to turn these cells on and off for therapeutic purposes,” Dr. Dixon said.
Drs. Dixon and Prlic are studying how inflammation is regulated in oral tissues, but the implications are wide-ranging. Chronic inflammation has been implicated in many illnesses ranging from gingivitis to heart disease. However, Dr. Dixon said, inflammation is not necessarily a bad thing at a sufficiently low level. “There’s always a background level of inflammation in the mouth and the gut,” he said, adding that it’s actually a protective “sentinel state.”
“But some patients’ immune system just can’t resolve or respond appropriately during an infection, and we’re trying to figure that out,” he said. “You can’t have an unbridled immune response – there would be massive tissue destruction. The challenge is to know how to turn immune cell functions off and on as necessary.”
Using their discoveries, Drs. Dixon and Prlic recently applied for additional National Institutes of Health funding to support the development of therapies to halt chronic inflammation and induce tissue repair.
“I am excited to keep working with Doug on trying to figure out how we can best tweak the immune response to resolve inflammation and enhance tissue repair,” Dr. Prlic said.